Mission.
We drive the mainstream adoption of digital technology in healthcare
We believe emerging digital health technology is the future of healthcare. There is no substitute for safety and efficacy, and we believe there is a hybrid solution for every medical problem; one that benefits from the amazing possibilities offered by digital health technology, and the necessary rigour demanded of all health solutions.
Learn more
Working backstage with some of the most exciting
digital health organizations in the world

Digital technology is only the beginning
Great technology alone cannot solve
complex healthcare challenges
The healthcare industry is undergoing an innovation disruption that demands an experienced and collaborative approach. Many requirements must be met before a great digital health solution can fill clinical gaps and lead to positive health outcomes.
-
1.
Technology
Medical device rigour with agile, patient-centric development.
-
2.
Clinical Applications
Validated solutions targeted at real clinical use cases.
-
3.
Regulatory Strategy
The right level of compliance to meet the needs at all stages of development.
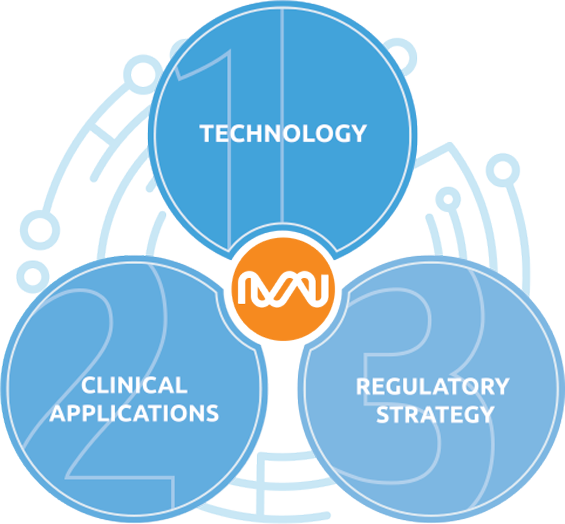
All three must be integrated to support a real go-to-market strategy through payers, providers and health programs. How does your solution measure up?
See how you measure up
Services.
BML Health offers a full range of services to the digital health ecosystem
Digital health startups
The runway is long and cash burn is high. There isn’t a lot of room for mistakes. We take a special interest in digital health startups and provide customized services to this emerging field.
Learn moreTechnology companies
We work with cutting-edge technology companies to help them get their tech right, design and validate the best metrics and target adoption by making real changes to health, wellness and performance.
Learn moreClinical & research organizations
We work with medical professionals, health services providers, life sciences companies and world-class clinical and research organizations to support development of digital health solutions with real clinical application in precision medicine and personalized care.
Learn moreOther health stakeholders
We help investor groups, insurance companies, health plan providers, government organizations and others, to evaluate digital health technology and its potential impact in healthcare.
Learn moreTrusted by.
Our partners.
-
VisCardia is thrilled to share recent news that FDA approved our application for IDE, to move forward with a US Clinical Study. This confirms there is sufficient data to protect study subjects with symptomatic Heart Failure, and an expectation that the benefits of VisONE Therapy exceed any known risks. Thank you for BML’s help strategizing, guiding, and authoring [responses to FDA submission review questions]. It really takes a village to achieve such an impactful milestone, and we couldn’t have done it without you. And thank you for celebrating this milestone with us! It’s a true testament to BML’s world-class expertise
- Carin Berg, Assistant Technical Program Manager -
BML Health understands my way of thinking, my needs and they can translate them into regulatory strategy with the FDA and Health Canada very rapidly. BML is easy to work with, you feel like you're speaking to members of your team. Working with them is an enjoyable experience. I recommend BML to other firms all the time. To me Marc is the premier independent regulatory consultant in Quebec and maybe in Canada.
- Steven Arless , Serial Medtech Entrepreneur -
From the very beginning, BML guided us in developing the most relevant regulatory strategy for our medical device, in accordance with the requirements of the FDA and Health Canada. We also benefited from their support in the development of major clinical trials with hospital centers. Today, thanks to their extensive experience in medical device development, their team plays a crucial role in managing the stakeholders involved in the design and development of our products. But above all, we appreciate working with a passionate, driven, and caring team who goes above and beyond to help us achieve our goals as a medtech company.
- Nanette Sene, Co-Founder and CEO -
From my first interactions with Marc, it was clear to me that BML was a great fit for us and would become a trusted partner moving forward. He and his team have locked on to our strategy and mission, and are implicated in a fundamental way. They have become an integral part of our team at several levels, as we navigate the complexities of medical software regulation and compliance in both Canada and the US. I think the main thing that sets them apart is the holistic approach, as they consider the clinical application and the business as much as the compliance and regulatory aspects that are their mandate. Working with them has challenged us to dig deep and clearly define our use case and essentially our clinical service offering, not only to Health Canada and the FDA, but to all stakeholders. Beyond strategy, BML has provided tremendous tactical support toward the ongoing improvements to our ISO13485-certified quality management system and how to apply it to the development of our AI-based medical devices in a scalable manner.
- Mara Lederman, COO -
Marc and George bring the utmost professionalism to every discussion and working meeting we have had throughout the Proteocyte breakthrough device submission process with the FDA. After a few discussions, I found it hard to believe they were new to our product, because they expressed a deep understanding of the scientific literature and of the cancer-prediction diagnostic space. They are meticulous wordsmiths, listening carefully to our comments and incorporating them into the documents with little of the time consuming back-and-forth discussions; we are more efficient and effective with BML as part of our team. Their decades of experience, coupled with intense attention to detail, has made working with them a pleasure.
- Dr Antony Morlandt, CEO -
BML has been essential in helping us organize our team and software development, understand how to properly document our software to meet the requirements of various certifications, and work more efficiently. BML has advised us in validating our product compliance with Health Canada and in the implementation of medical software design control management. Joanna has been an outstanding collaborator: we appreciate the good availability, understanding, knowledge, quick problem solving and her excellent interpersonal treatment.
- Nicolas Jimenez, Co-Founder and CEO -
BML has been a highly valued partner in Aifred Health’s journey toward Software as a Medical Device compliance. Marc provided impactful, hands-on support to the Aifred leadership team over a period of several months, helping us to understand, navigate and coordinate complex requirements across multiple functions. He became an invaluable extension of our team during this time, flexible and responsive to our needs, wholly committed to helping the team get up the learning curve, and dedicated in his support.
- Marina Massingham, CEO -
BML was our trusted partner to get our smart textile platform ready for the medical field. It was important for us to have independent 3rd party validation of our technology and to have access to an experienced team to prepare us for digital health applications. BML's expertise and network were invaluable to get our technology ready and to set up a winning strategy for the medical space. Marc and his team were always professional, insightful and helped win landmark projects for OMsignal.
- Fred Chanay, CEO