Short Answer: No.
Well, ok…. kind of. But not exactly.
It can do something that only one other over-the-counter device can do, and that’s to measure medical grade ECG without a physician or certified technician (more on the other device below).
Confusion is already spreading and some interesting points in favor and against are being raised daily. I’ll do my best to answer a few of the questions I have received since the launch.
Is the Apple Watch ECG the same as medical ECG?
Again… kind of. The medical ECG (which is the abbreviation for electrocardiogram, i.e. the recording of the electrical activity of your heart) you receive in a hospital or clinic from a physician or certified technician normally requires several more electrodes than there are on the watch. These measure your heart from multiple angles, allowing a more complete picture of your heart’s activity and thus numerous and more complex diagnoses. They also help to protect the recording from unwanted noise. You may have heard of a 12-lead or 5-lead ECG; in these configurations, the number of leads is related to the number of electrodes placed on the skin. The more electrodes you use, the more leads (or angles) you can measure.
To further add to the confusion, the term ECG is often used to refer to the collective which includes the recording, the type of test required (e.g. routine ECG, cardiopulmonary exercise test, stress test, Holter, etc) and the report that makes it into your doctor’s hands.
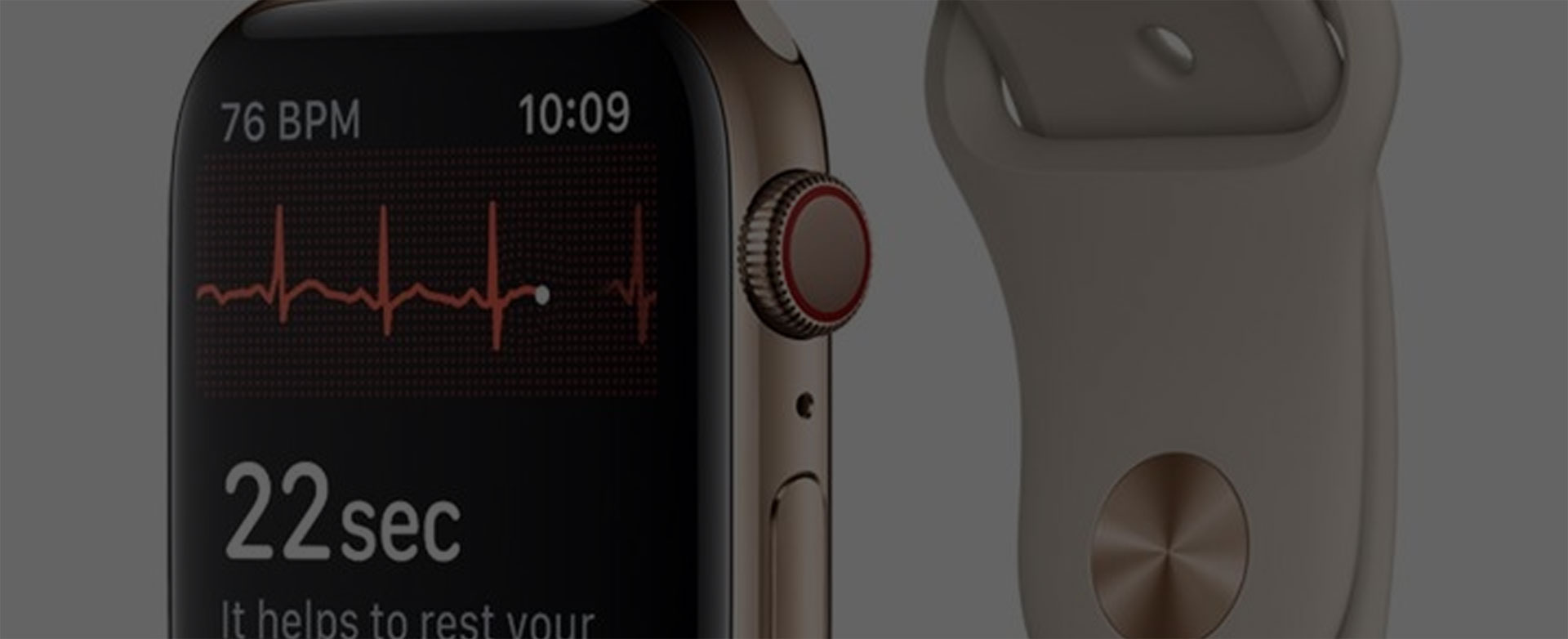
The Apple watch only measures one “lead”, which is a rudimentary version of the more complex medical systems (note that there are other single and dual-lead ECG devices registered with the FDA as medical devices). It also measures an ECG tracing in no particular context, and whenever the user decides to make a recording.
The resulting report or metrics displayed to the user are as of yet unknown, although we have been given a few hints. Apple is claiming to detect atrial fibrillation (a.k.a. AFib), which is a precursor to heart failure (i.e cardiac arrest), blood clots and even stroke. The FDA is allowing Apple to claim that it can detect AFib but not well enough to make a medical decision, rather only to recommend a visit to your cardiologist’s office, and only under certain conditions.
An interesting detail about AFib is that roughly one third of the approximately 35 million people worldwide with AFib are actually asymptomatic (a condition called silent AFib). These individuals exhibit no outward effects of their abnormal cardiac activity, and have had it diagnosed almost accidentally, perhaps during a routine ECG or some other test. In a regular medical context, if silent AFib is suspected, long-term monitoring with a Holter monitor is prescribed. And even then, if the sporadic event does not occur during the monitoring period, it remains undetected and the condition remains undiagnosed.
The Holter testing tends to be on the order of 24 hours. The Apple Watch measurement is 30 seconds at a time.
So in short, the Apple watch is a single-lead ECG that can be used to detect (not diagnose) AFib activity. Maybe.
What’s the difference between this new watch and the previous versions?
As mentioned, the ECG is a recording of the electrical activity of the heart. Photo-plethysmography (or PPG) is the sensing of blood flow through an artery using Infrared (IR) technology. While both can be used to estimate heart rate (HR), they are very different in nature, technology and underlying physiology. The Apple Watch 3 uses PPG technology to measure the pulse of blood flow in your wrist, and it does so the entire time you are wearing the watch.
The new watch measures ECG. It includes an extra electrode on the crown, on which the user places the finger of their opposite hand when they want to make a recording. This provides a measurement across the heart which is required to capture the ECG signal. In this case, it is from the wrist of one hand to the finger of the other. (This is a placement derived from what is referred to as wrist-to-wrist, in which an electrode or strap is placed on both wrists and the signal is measured between the two).
ECG is the gold standard for measurement of cardiac activity in medical applications. It is generally less susceptible to noise and movement artifact than PPG. With the new watch, the user is instructed to sit still and record without moving for 30 seconds at a time.
At rest, PPG offers a fast, simple way to capture blood flow and estimate heart rate. In a medical setting, PPG is used exclusively on the finger of resting patients as a fast, easy way to display HR. You’ve seen this in every show or movie when a character is hospitalized and their vitals are taken from a finger clip and displayed on a bedside monitor.
You’ve seen this in every show or movie when a character is hospitalized and their vitals are taken from a finger clip and displayed on a bedside monitor
In recent years, PPG has become popular in wearable devices like smartwatches to provide an approximation of HR in lifestyle and active contexts. The main reason is the rapid adoption of wrist-worn devices over most other form factors, which is due mainly to a choice of taste and style over performance. Unfortunately, the PPG signal at the wrist (and from any other placement location other than the finger, in fact) is not the most reliable, and it is far too susceptible to movement and other artifacts (which lead to erroneous values of HR) to be used reliably in any medical context.
These artifacts lead to gaps in coverage, which can occur for seconds or minutes at a time and involve significant errors that are often hidden from the user. Researchers at the Cleveland Clinic, led by Dr Marc Gillinov of the Judith Dion Pyle Chair in Heart Valve Research, Thoracic and Cardiovascular Surgery, found:
“ …wrist-worn [PPG] monitors are less accurate than the standard chest strap [ECG]. We found these devices can equally over- and underestimate heart rate. The error ranged from +/-34 beats per minute to +/-15 beats per minute, depending on the type of activity.“
It looks like the new watch includes both the PPG from the wrist and the ECG when the finger is placed on the crown. The engineer in me wants to know whether both can be done at the same time, and if so, whether they report the same heart rate.
Does it even work?
Definitely. Wrist-to-wrist (or wrist-to-finger) ECG is relatively easy to acquire. The myriad of issues related to movement, artifacts and outcomes have been handled by Apple with a very limited use case: sit, record, stop, ask your doctor. What Apple has done, as it tends to do so well, is create a UX and a story that delivers the whole solution in an elegant, stylish package for consumers.
The new watch isn’t a Holter monitor and it isn’t a wrist worn fitness monitor. There are only certain conditions under which it will work well, and it is still neither recommended nor intended to replace a medical ECG. It is a medical-grade portable single-lead ECG that comes with limitations and clear instructions for use (as mandated by the FDA). The recent Gizmodo article entitled “Is the New Apple Watch’s ECG Feature Legit?” sheds a little light on the limitations.
Essentially, users will need to be well-informed and expectations will have to be managed.
The other over-the-counter device registered by the FDA as an ECG is the Kardiaband from AliveCor. Not coincidentally, it is an accessory band that was designed and sold for use with the Apple Watch 3, and it works the same way as the new watch: the user places the finger of the opposite hand on the watch band and wrist-to-finger ECG is captured. It was registered as an ECG device with the FDA a few years ago. So we know the technology works.
Not to be outdone, AliveCor just announced they will be releasing a 6-lead device for iPhone that can detect up to 100 heart diseases (and according to 9to5Mac, this new product is protected by several patents so AliveCor should be just fine), while the new Apple Watch is designed to detect only AFib. As the offering grows in breadth, Apple will need to update their FDA registration to include new conditions. This of course is doable, but it takes more time and requires more scrutiny than unregulated development.
How will this impact health care and health care services?
I will stick to the tech and let the health care business leaders and analysts postulate, project and comment as they are wont to do. One interesting take on it from Wired, entitled “The ECG in the new Apple Watch is a potential healthcare headache” raises predictable concerns about clogging emergency rooms, in particular, and other general issues.
This is only the beginning of the debate. Time will tell if this is a gadget or a health care revolution in the making. But one thing is sure…
…while the rest of us nitpick and scramble to make sense of it, Apple has just changed the conversation. Again.
The future should be fun.
For now, there is still confusion. A quick search on the FDA website for “Apple Watch Series 4” reveals a series of posts about arsenic poisoning in apple juice. This, of course, may just be an artifact.
References
- Gillinov S1, Etiwy M, Wang R, Blackburn G, Phelan D, Gillinov AM, Houghtaling P, Javadikasgari H, Desai MY. Variable Accuracy of Wearable Heart Rate Monitors during Aerobic Exercise. Med Sci Sports Exerc. 2017 Aug;49(8):1697-1703
- https://gizmodo.com/is-the-new-apple-watchs-ecg-feature-legit-1829010308
- https://www.wired.co.uk/article/apple-watch-heart-ecg-fda
- https://search.usa.gov/search?utf8=%E2%9C%93&affiliate=fda&query=apple+watch+series+4